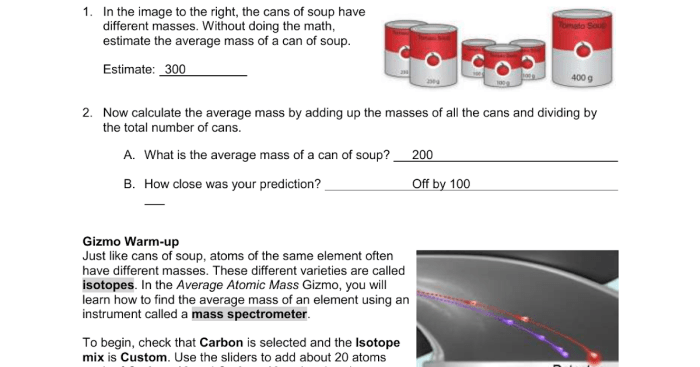
Student exploration average atomic mass – Delving into the realm of chemistry, the concept of average atomic mass emerges as a fundamental pillar, playing a pivotal role in understanding the behavior and properties of elements. This exploration embarks on a journey to unravel the intricacies of average atomic mass, its significance, and its multifaceted applications.
The subsequent paragraphs will delve into the intricacies of calculating average atomic mass, examining the factors that influence its variations, and exploring its practical applications in various scientific disciplines. By the conclusion, a comprehensive understanding of average atomic mass will be attained, empowering individuals to navigate the complexities of chemistry with greater confidence.
Atomic Mass and Its Significance
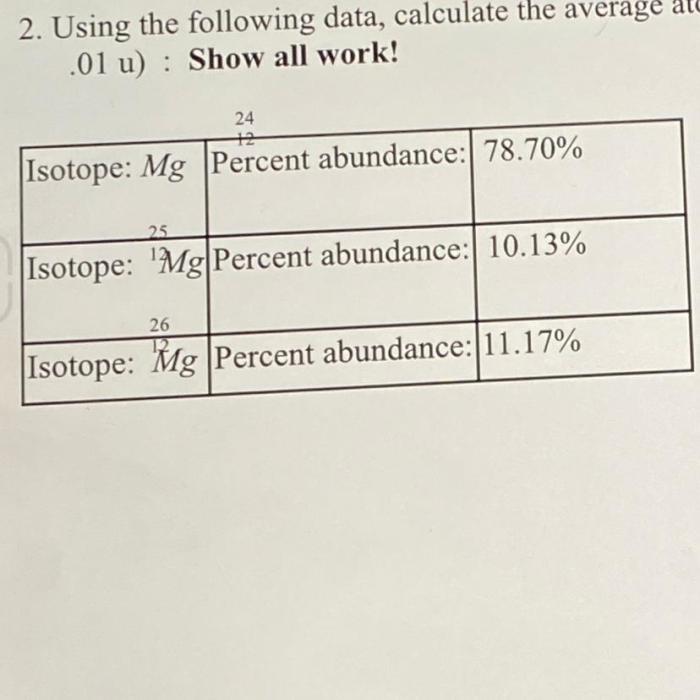
Atomic mass is a fundamental property of an element, representing the average mass of its atoms. It is calculated as the weighted average of the masses of all naturally occurring isotopes of the element, considering their relative abundances.
Atomic mass plays a crucial role in chemistry. It is used to determine the molar mass of compounds, which is essential for stoichiometric calculations and predicting chemical reactions. Additionally, atomic mass provides insights into the nuclear structure and stability of isotopes, aiding in the understanding of nuclear chemistry and radioactive decay.
Calculating Average Atomic Mass
The average atomic mass of an element is calculated using the following formula:
Average Atomic Mass = Σ(Abundance of Isotope × Mass of Isotope)
Where:
- Abundance of Isotope refers to the percentage of the specific isotope in the natural mixture.
- Mass of Isotope is the mass of the individual isotope, typically expressed in atomic mass units (amu).
By multiplying the abundance of each isotope by its mass and summing the products, we obtain the average atomic mass of the element.
Factors Affecting Average Atomic Mass
The average atomic mass of an element can be influenced by several factors, including:
- Isotopic Composition:The relative abundance of different isotopes within an element can vary, leading to variations in average atomic mass.
- Natural Variations:Natural processes, such as radioactive decay and geological fractionation, can cause variations in isotopic composition and, consequently, average atomic mass.
Applications of Average Atomic Mass
Average atomic mass has numerous practical applications in various fields:
- Molecular Weight Determination:Average atomic mass is used to calculate the molecular weight of compounds, which is crucial for understanding their properties and behavior.
- Property Prediction:The average atomic mass of an element can provide insights into the physical and chemical properties of its compounds.
- Research and Industry:Average atomic mass is utilized in various research and industrial applications, including nuclear chemistry, geochemistry, and materials science.
Limitations of Average Atomic Mass, Student exploration average atomic mass
While average atomic mass provides valuable information, it has certain limitations:
- Isotopic Ratio Variability:In certain applications, the isotopic ratio of an element may vary significantly, making average atomic mass less representative.
- Isotopic Information Loss:Average atomic mass does not provide detailed information about the individual isotopes present or their relative abundances.
General Inquiries: Student Exploration Average Atomic Mass
What is the significance of average atomic mass?
Average atomic mass is a weighted average of the masses of all the isotopes of an element, taking into account their relative abundances. It provides a representative value for the mass of an element’s atoms, which is crucial for various chemical calculations.
How is average atomic mass calculated?
Average atomic mass is calculated by multiplying the mass of each isotope by its abundance and then summing the products. The abundance of each isotope is typically expressed as a percentage or a fraction.
What factors can affect the average atomic mass of an element?
The average atomic mass of an element can be affected by variations in the isotopic composition of the element. Natural variations in the abundance of isotopes can lead to different average atomic masses for the same element from different sources.